Facility of Multimodal Imaging - AMMI Maastricht, The Netherlands
The Advanced Microscopy and Molecular Imaging (AMMI) Node connects state-of-the-art (light and electron) microscopy and mass spectroscopy-based innovative molecular imaging with high-end, non-invasive (bio)medical imaging technologies. It aims to assist academic and industrial users performing fundamental and applied studies in biological and biomedical (molecular) imaging. The AMMI infrastructure contains both basic and high-end/one-of-a-kind imaging techniques on microscopic and macroscopic level combined with an extensive palette of non-invasive imaging techniques.
Read the news from the AMMI Node
Specialties and expertise of the Maastricht Node
The Advanced Microscopy and Molecular Imaging (AMMI) facility offers state-of-the-art (light and electron) microscopy, mass spectrometry-based molecular imaging, and non-invasive pre-clinical and clinical imaging technologies. AMMI aims to provide diagnostic and prognostic imaging technologies for personalized medicine. As such, AMMI offers access to a translational, interdisciplinary research program in leading international expertise centers, combining research and education.
Via our world-leading local Research Schools, our imaging platform covers a broad palette of research topics as resulting from collaborations on cardiovascular, oncological, orthopedics, metabolic, neurological, and regenerative medicine research. For more information, see link.
Offered Technologies:
| Technologies | Euro-BioImaging |
| Laser scanning confocal microscopy (LSCM/CLSM) | ✓ |
| Spinning disk confocal microscopy (SDCM) | ✓ |
| Two-photon microscopy (2P) | ✓ |
| Stimulated emission depletion microscopy (STED) | ✓ |
| Second/Third Harmonic Generation | ✓ |
| High throughput microscopy/high content screening (HTM/HCS) | ✓ |
| Intravital Microscopy (IVM) | ✓ |
| Tissue Clearing * | ✓ |
| Fluorescence Resonance Energy Transfer (FRET) | ✓ |
| Fluorescence Recovery After Photobleaching (FRAP) | ✓ |
| Fluorescence Lifetime Imaging Microscopy (FLIM) | ✓ |
| micro-MRI/MRS (>= 7T) | ✓ |
| micro-MRI/MRS (< 7T) | ✓ |
| micro-CT | ✓ |
| micro-PET | ✓ |
| micro-SPECT | ✓ |
| micro-US | ✓ |
| micro-PET/MRI | ✓ |
| micro-PET/CT | ✓ |
| micro-MRI/MRS (>= 7T) - ex-vivo | ✓ |
| micro-MRI/MRS (< 7T) - ex-vivo | ✓ |
| micro-CT - ex-vivo | ✓ |
| Mass spectrometry-based imaging (MSI) - med* | ✓ |
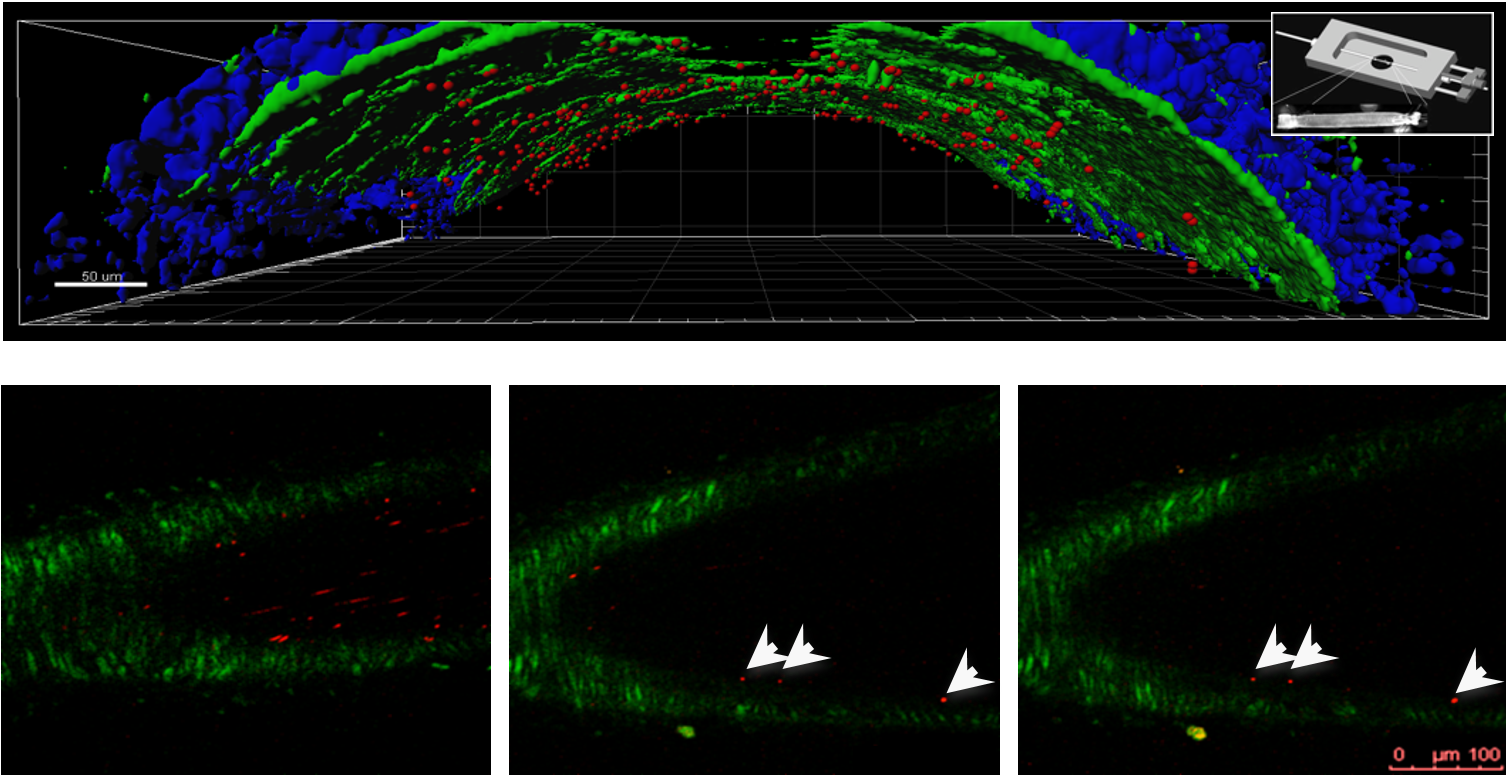 Example of Two-photon microscopy: Imaging of the attachment of anti-ICAM-1 bimodal (US-TPLSM) bubbles to damaged carotid artery ex vivo (top) and in vivo (bottom) For more information see: Noninvasive molecular ultrasound monitoring of vessel healing after intravascular surgical procedures in preclinical setup. A. Baleanu-Curaj, Z. Wu, T. Lammers, C. Weber, M.A.M.J. van Zandvoort, and F. Kiessling Arterioscler Thromb Vasc Biol 2015, 35(6): 1366-1373. DOI: 10.1161/ATVBAHA.114.304857
Example of Two-photon microscopy: Imaging of the attachment of anti-ICAM-1 bimodal (US-TPLSM) bubbles to damaged carotid artery ex vivo (top) and in vivo (bottom) For more information see: Noninvasive molecular ultrasound monitoring of vessel healing after intravascular surgical procedures in preclinical setup. A. Baleanu-Curaj, Z. Wu, T. Lammers, C. Weber, M.A.M.J. van Zandvoort, and F. Kiessling Arterioscler Thromb Vasc Biol 2015, 35(6): 1366-1373. DOI: 10.1161/ATVBAHA.114.304857
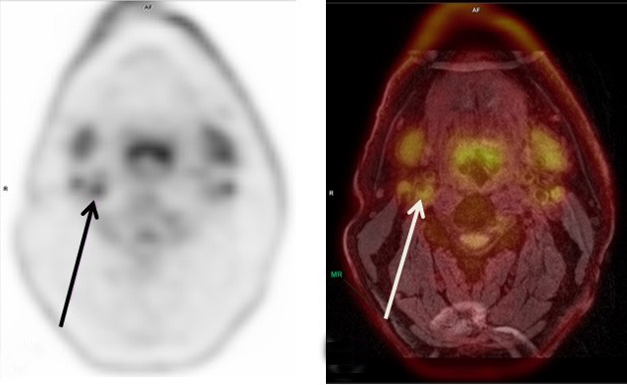 Example of PET/MRI imaging: 18F-FDG PET image of the neck (left arrow) of a patient with a carotid plaque; (B) a color overlay of the PET image as displayed in (A) on the corresponding MR image in (B) For more information see: PET/MRI of atherosclerosis. M. Aizaz, R. Moonen, J. van der Pol, C. Prieto, R. Botnar and M. Kooi Cardiovasc Diagn Ther 2020;10(4):1120-1139. DOI: cdt.2020.02.09
Example of PET/MRI imaging: 18F-FDG PET image of the neck (left arrow) of a patient with a carotid plaque; (B) a color overlay of the PET image as displayed in (A) on the corresponding MR image in (B) For more information see: PET/MRI of atherosclerosis. M. Aizaz, R. Moonen, J. van der Pol, C. Prieto, R. Botnar and M. Kooi Cardiovasc Diagn Ther 2020;10(4):1120-1139. DOI: cdt.2020.02.09
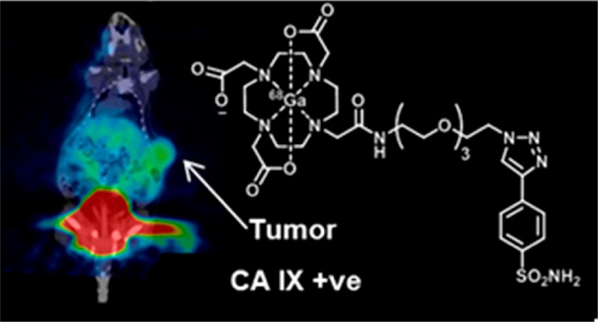 Example of microPET/CT fusion imaging in a tumour-bearing mouse For more information see: Synthesis and in Vivo Biological Evaluation of (68)Ga-Labeled Carbonic Anhydrase IX Targeting Small Molecules for Positron Emission Tomography. D. Sneddon, R. Niemans, M. Bauwens, A. Yaromina, S. van Kuijk, N. Lieuwes, R. Biemans, I. Pooters, P. Pellegrini, N. Lengkeek, I. Greguric, K. Tonissen, C. Supuran, P. Lambin, L. Dubois and S. Poulsen Journal of Medicinal Chemistry, 29 Jun 2016, 59(13):6431-6443. DOI: 10.1021/acs.jmedchem.6b00623 https://radiomicsimagingarchiv...
Example of microPET/CT fusion imaging in a tumour-bearing mouse For more information see: Synthesis and in Vivo Biological Evaluation of (68)Ga-Labeled Carbonic Anhydrase IX Targeting Small Molecules for Positron Emission Tomography. D. Sneddon, R. Niemans, M. Bauwens, A. Yaromina, S. van Kuijk, N. Lieuwes, R. Biemans, I. Pooters, P. Pellegrini, N. Lengkeek, I. Greguric, K. Tonissen, C. Supuran, P. Lambin, L. Dubois and S. Poulsen Journal of Medicinal Chemistry, 29 Jun 2016, 59(13):6431-6443. DOI: 10.1021/acs.jmedchem.6b00623 https://radiomicsimagingarchiv...
Additional services offered by the Node
- Technical assistance (running instruments, probe preparation, sample preparation)
- Methodological setup (design of study protocol and standard operation procedures)
- Training in infrastructure use
- Wet lab space including biological material storage and processing possibilities
- Animal breeding, ethical licenses, experiment protocols
- Animal holders for clinical devices
- Server space, data processing and analysis (AI, deep learning and radiomics)
- Training workstations and seminar rooms
- Cryo-sectioning
- Image (CT and BLI)-guided small animal irradiator
Through collaboration we also offer the possibility to use various other advanced microscopy techniques at different close-by labs (e.g. Uniklinik Aachen), such as animal whole-body fluorescence imaging, Lightsheet, Rescan Confocal, and Two-photon intravital and lifetime microscopy.
Instrument highlights
With this broad range of techniques, we have created an imaging platform, ranging from EM, via super-resolution STED (592-depletion, resonant scanning), confocal (resonant scanning, white-light laser, in vivo cell system), and multi-photon (intravital and lifetime included) microscopy, towards whole body imaging. This platform connects a broad range of microscopic and macroscopic imaging techniques. Some of the EM and LM techniques are located in the Microscopy Core Lab (MCL) run by Dr. C. Lopez-Iglesias.
Furthermore, the Node provides access to the Mass Spectrometry Imaging CORE laboratory, as run by Dr. B. Cillero Pastor. Technical features include:
- High throughput, high spatial resolution, and high mass resolution MALDI imaging
- MALDI 2-postionization technology
- Isomeric resolution by ozone‐induced dissociation
- Nano-ToF-SIMS for biomolecular imaging at single cell level
- i-Knife technology for real time tissue classification
- Laser microdissection capabilities
- Label free proteomics
- Native MS
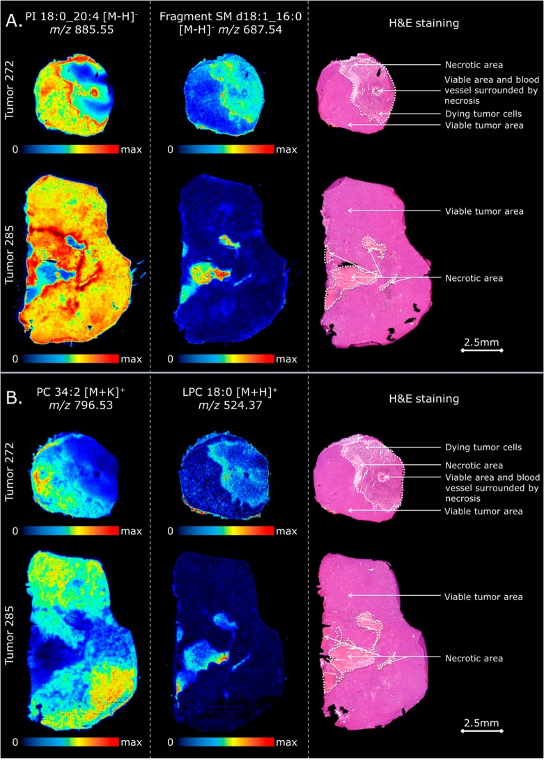 Example of MALDI imaging: Molecular visualization of intratumor heterogeneity For more information see: Specific Lipid and Metabolic Profiles of R-CHOP-Resistant Diffuse Large B-Cell Lymphoma Elucidated by Matrix-Assisted Laser Desorption Ionization, Mass Spectrometry Imaging and in Vivo Imaging. F.P.Y. Barré, B.S.R. Claes, F. Dewez, C. Peutz-Kootstra, H.F. Munch-Petersen, K. Grønbæk, A.H. Lund, R.M.A. Heeren, C. Côme and B. Cillero-Pastor Anal Chem. 2018, 90(24):14198-14206. DOI: 10.1021/acs.analchem.8b02910
Example of MALDI imaging: Molecular visualization of intratumor heterogeneity For more information see: Specific Lipid and Metabolic Profiles of R-CHOP-Resistant Diffuse Large B-Cell Lymphoma Elucidated by Matrix-Assisted Laser Desorption Ionization, Mass Spectrometry Imaging and in Vivo Imaging. F.P.Y. Barré, B.S.R. Claes, F. Dewez, C. Peutz-Kootstra, H.F. Munch-Petersen, K. Grønbæk, A.H. Lund, R.M.A. Heeren, C. Côme and B. Cillero-Pastor Anal Chem. 2018, 90(24):14198-14206. DOI: 10.1021/acs.analchem.8b02910
Contact details
Node leader pre-clinical techniques:
Marc A.M.J. van Zandvoort, Professor in Advanced Microscopy
mamj.vanzandvoort@maastrichtuniversity.nl
+31 (0)43 388 1998
Node-leader non-invasive techniques:
Ludwig Dubois, Associate Professor Precision Medicine
Ludwig.dubois@maastrichtuniversity.nl
+31 (0)43 388 2909
Proof of Concept Node-leader Mass Spectroscopy Imaging:
Michiel Vandenbosch, CORE lab leader
m.vandenbosch@maastrichtuniversity.nl